from http://www.socialsecurityhome.com/disabilityblog/2009/03/07/292/
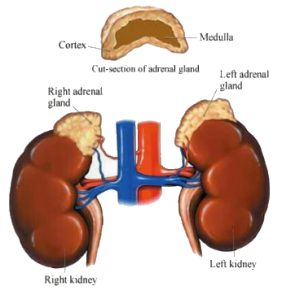
Addison’s disease is also called adrenal insufficiency, adrenocortical hypofunction, and hypocortisolism. Addison’s disease is a disease that affects your adrenal glands. Your adrenal glands are located right above your kidneys. The outside layer of these glands make hormones that help your body regulate your salt and water balance and your blood pressure. These hormones also help your body respond to stress. Addison’s disease occurs when your adrenal glands do not make enough of these hormones.
If you, your spouse, or your child with disability has been diagnosed with Addison’s disease, you may be in need of financial help. This may be especially true if Addison’s disease has become so serious a problem that it is the reason for the disability of you, your spouse, or your child with disability.
In fact, you may have applied for a social security disability benefit or disability benefit from the social security administration because of the disability caused by Addison’s disease.
If you were denied, and are planning on reapplying or appealing the denial by the social security administration, you will need the help and skill of an experienced disability lawyer who will work with you through this involved procedure.
This is a Web site where you can find a capable disability lawyer. The expert disability lawyer who will work with you or your spouse through SocialSecurityHome.com can assist you in reapplying or appealing the denial for a social security disability benefit or disability benefit for you, your spouse, or your child with disability because of the disability caused by Addison’s disease.
It will be good for you to know what you can about Addison’s disease. The more you know about the condition that you have, the better prepared you will be to deal and get help with Addison’s disease.
Addison’s disease is the failure of your adrenal glands to produce certain hormones. These hormones give instructions to nearly every tissue and organ in your body. Cortisol is one of the hormones not produced sufficiently in Addison’s disease. Aldosterone is another hormone that there is too little of.
Addison’s disease can happen at any age of life. It usually occurs, however, in people between the ages of 30 and 50.
The symptoms and signs of Addison’s disease may appear slowly over a period of several months. Some of these indications of Addison’s disease are:
* Craving salt
* Irritability
* Depression
* Vomiting, nausea, or diarrhea
* Low blood sugar (hypoglycemia)
* Muscle fatigue and weakness
* Decreased appetite and weight loss
* Low blood pressure and possible fainting
* Skin darkening (hyperpigmentation).
There are times, however, when the signs and symptoms of Addison’s disease can manifest themselves suddenly. This happens with acute adrenal failure (addisonian crisis). This may involve additional signs and symptoms like:
* Low blood pressure
* Loss of consciousness
* Pain in your abdomen, legs, or lower back
* Severe diarrhea and vomiting, resulting in dehydration.
The most usual cause of Addison’s disease is the body attacking itself (autoimmune disease). For some unknown reason your immune system looks at the outer layer (cortex) of your adrenal glands that produces essential hormones as something foreign to be attacked and destroyed. Other possible causes of Addison’s disease are:
* Cancer spread to the adrenal glands
* Tuberculosis
* Bleeding into the adrenal glands
* Other infections of the adrenal glands.
The above listed things are possible causes of what doctors term primary adrenal insufficiency. There is also what is termed secondary adrenal insufficiency. This is caused by the failure of your pituitary gland to produce a hormone that stimulates the adrenal cortex to produce its hormones. This can result in your adrenal cortex failing to produce its hormones even though your adrenal glands are not damaged. This is what doctors call secondary adrenal insufficiency. Another more likely cause of secondary adrenal insufficiency happens when you are taking corticosteroids for the treatment of chronic conditions like arthritis or asthma, and you abruptly stop taking them.
Your doctor will probably ask you about your signs and symptoms and your medical history. If your doctor thinks you may have Addison’s disease there are several tests you may be asked to take. Some of these may include imaging tests, blood test, insulin-induced hypoglycemia test, and ACTH stimulation test. All of this will help your doctor to diagnose Addison’s disease.
Treatment for your Addison’s disease if diagnosed early may involve taking prescription corticosteroids. Your doctor may also want you to take one or more hormones that your body is not producing sufficiently. These are usually taken in amounts that are what the body would normally produce. Stressful situations like an infection, minor illness, or an impending operation may require a temporary increase in your dosages.
One of the dangers associated with Addison’s disease is an addisonian crisis. This is a life-threatening situation that results in high blood levels of potassium, low blood pressure, and low blood sugar levels. An addisonian crisis is usually treated with intravenous injections of saline solution, hydrocortisone, and sugar (dextrose).
Hopefully, this information about Addison’s disease will be helpful in getting the assistance you, your spouse, or your child with disability needs because of your disability caused by Addison’s disease.
As mentioned at the beginning, if you intend to apply for a social security disability benefit or disability benefit because of the disability caused by Addison’s disease, or you have already applied and been turned down, and you plan on reapplying or appealing the denial by the social security administration; you will need to enlist a competent disability lawyer to help and guide you through this process.
This is the right Web site for finding a skilled disability lawyer. The expert disability lawyer at SocialSecurityHome.com who will work with you or your spouse can help you in your claim for a social security disability benefit or disability benefit because of the disability caused by Addison’s disease.
This is something important for you, your spouse, or your child with disability. Do not put this off. Contact a skilled disability lawyer at SocialSecurityHome.com today.
Tags: Addison's disease, Disability Attorney, Social Security Disability, Social Security Disability Lawyer