This article is written live from the American Association of Clinical Endocrinologists (AACE) 2017 Annual Meeting in Austin, TX. MPR will be reporting news on the latest findings from leading experts in endocrinology. Check back for more news from AACE 2017.
Contributors
Discussions
Categories
- 17a-OH-Progesterone (1)
- 24-hour urine free cortisol (1)
- 24-hour urine test (3)
- abbreviations (1)
- ABC TV (2)
- abstract (5)
- acne (3)
- acromegaly (4)
- ACTH (18)
- activist (1)
- Addison-Biermer disease (2)
- Addison-Schilder syndrome (2)
- Addison's (5)
- Addison's Disease (72)
- Addison’s disease (1)
- Addisonian anemia (1)
- Addisonian crisis (1)
- adenoma (17)
- adrenal (45)
- adrenal cortex (1)
- Adrenal Crisis (33)
- Adrenal Fatigue (8)
- adrenal glands (84)
- Adrenal Insufficiency (58)
- adrenal medulla (1)
- adrenal vein sampling (1)
- adrenal venous sampling (1)
- adrenal-sparing (1)
- adrenalectomy (25)
- adrenaline (1)
- adrenocortical carcinoma (3)
- Adrenoleukodystrophy (1)
- AI (1)
- aldosterone (10)
- aldosteronism (2)
- Alice (2)
- AMRC5 mutation (1)
- androgen (2)
- anniversary (1)
- anxiety (1)
- app (1)
- appendicitis (1)
- April Fools (1)
- arthritis (3)
- autoimmune (1)
- AVIS (1)
- AVS (1)
- award (1)
- awareness (8)
- BclI polymorphism (1)
- benign (3)
- bilateral (2)
- bios (3)
- birthday (2)
- BLA (5)
- blogger (4)
- blogs (10)
- BlogTalkRadio (2)
- blood (2)
- blood pressure (4)
- blood test (2)
- Blue and Yellow (1)
- body image (1)
- bone loss (1)
- books (1)
- brochures (1)
- bruising (2)
- buffalo hump (1)
- Cabergoline (1)
- CAH (6)
- cancer (5)
- Capitol Hill (1)
- carcinoma (1)
- cardiac (1)
- cardiovascular (1)
- Carney Complex (1)
- celiac (3)
- chat (1)
- childhood (1)
- children (2)
- chronic fatigue (1)
- chronic illness (1)
- Chronocort (1)
- chronotherapy (1)
- circadian rhythm (1)
- Cleveland Clinic (2)
- clinical trial (3)
- clinical trials (2)
- comments (1)
- conference (4)
- conference call (1)
- Congenital adrenal hyperplasia (8)
- Congress (1)
- Conn Syndrome (1)
- Conn's Syndrome (3)
- COR-003 (1)
- Corcept (6)
- Corlux (5)
- CORT 108297 (1)
- cortef (3)
- Cortendo AB (1)
- corticosteroids (2)
- corticotropin (3)
- cortisol (14)
- cortisone (6)
- CRH (3)
- Cushie (2)
- Cushie Bookstore (2)
- CushieWiki (6)
- Cushing's (80)
- Cushing's Awareness Challenge (21)
- Cushing's Awareness Day (9)
- Cushing's Disease (15)
- Cushing's Syndrome (5)
- Cushings Help (11)
- CyberKnife (1)
- cyclic (1)
- depression (4)
- dexamethasone (2)
- dexametnosone (1)
- DHEA (3)
- diabetes (14)
- diabetes mellitus (3)
- diagnosis (1)
- disability (1)
- discussion (1)
- doctors (1)
- Donation (1)
- Dr. Adriana Ioachimescu (1)
- Dr. Alfredo Quinones-Hinojosa (1)
- Dr. Amir Hamrahian (2)
- Dr. Barbara Craven (1)
- Dr. Betul A. Hatipoglu (1)
- Dr. Beverly Biller (1)
- Dr. Daniel Kelly (1)
- Dr. David M. Cook (1)
- Dr. Edward Laws (1)
- Dr. Edward Oldfield (1)
- Dr. Harvey Cushing (1)
- Dr. Harvey Williams Cushing (1)
- Dr. James Findling (1)
- Dr. James Lind (1)
- Dr. Lynette Nieman (1)
- Dr. Maria Fleseriu (1)
- Dr. Nelson Oyesiku (1)
- Dr. Roberto Salvatori (2)
- Dr. Shlomo Melmed (1)
- Dr. Theodore Friedman (4)
- drugs (4)
- e-Patient Dave (1)
- Easter (1)
- ectopic (2)
- electrocardiogram (1)
- emergency (1)
- emergency room (1)
- endocrinologist (7)
- endoscopic (2)
- English (1)
- enogenous (1)
- epinephrine (1)
- ER (1)
- exercise (1)
- exophthalmos (1)
- familial (2)
- FAQ (2)
- fatigue (4)
- FDA (4)
- fibromyalgia (1)
- FIPA (1)
- forums (3)
- G-allele (1)
- ganglioneuroma (1)
- genetic (1)
- gland (1)
- Global Genes (1)
- glossary (1)
- glucocorticoid (1)
- glucocorticoids (5)
- glucocortoid (1)
- grand rounds (1)
- Graves' (1)
- Graves' Disease (1)
- Growth Hormone (4)
- haemochromatosis (2)
- Health Activist (1)
- Health Activist Hero award (1)
- Help Wanted (1)
- Helpful Hints (2)
- hemangioma (1)
- hemodialysis (1)
- hGH (2)
- hirsuitism (5)
- holiday (4)
- hormone (1)
- hormones (1)
- HPA axis (4)
- hydrocortisone (3)
- hyperaldosteronism (1)
- hypercortisolism (6)
- hyperkalemia (1)
- hyperplasia (1)
- hyperprolactinemia (1)
- hypertension (2)
- hyperthryoidism (1)
- hypopituitarism (1)
- hypothalamus (1)
- In Memory (3)
- incidentaloma (3)
- infertility (2)
- injection (1)
- insulin (2)
- Insulin Resistance (5)
- Insulin Tolerance Test (1)
- Interviews (7)
- iPad (1)
- iPhone (1)
- IPSS (4)
- Jane Austen (3)
- JFK (7)
- John Kennedy (8)
- Johns Hopkins (4)
- ketoconazole (6)
- kidney (2)
- kidney cancer (1)
- Korlym (11)
- laparoscopic (10)
- levothyroxine (1)
- libido (1)
- Lifetime TV (1)
- liver (1)
- Magic Foundation (1)
- map (1)
- Mary O'Connor (1)
- MaryO (7)
- Mayo Clinic (1)
- media (1)
- Medic Alert ID (1)
- Medical History (4)
- meds (1)
- meeting (2)
- MEN1 (2)
- MEN4 (1)
- message boards (4)
- metyrapone (1)
- midline incision (1)
- Mifepristone (5)
- mitotane (1)
- moon face (1)
- mortality (1)
- MRI (6)
- myelolipoma (1)
- Mystery Diagnosis (1)
- National Women's Health (1)
- Nelson's (1)
- Nelson's Syndrome (1)
- neurosteroids (1)
- neurosurgeon (1)
- News (1)
- news items (2)
- NIH (11)
- noradrenaline (1)
- noripinephrine (1)
- NormoCort (1)
- obese (1)
- obesity (7)
- ophthalmologist (1)
- Oprah (1)
- orphan disease (1)
- osteoporosis (3)
- Pasireotide (3)
- PCOS (3)
- perioperative (1)
- pernicious anemia (2)
- PharmaForm (1)
- pheochromocytoma (13)
- pituitary (52)
- Plenadren® (1)
- PMS (1)
- Podcast (7)
- potassium (3)
- Power Surge (3)
- PPNAD (1)
- prednisone (1)
- pregnancy (2)
- prevention (1)
- primary care (1)
- prizes (1)
- progesterone (1)
- prolactinoma (4)
- Psalm 116 (1)
- pseudocyst (1)
- psoriasis (1)
- quality of life (2)
- R-roscovitine (1)
- radiation (2)
- Rare Disease Congressional Caucus (1)
- Rare Disease Day (1)
- Rare Diseases (4)
- recurrence (2)
- renal artery (1)
- renal cell carcinoma (1)
- resolutions (1)
- retroperitoneal (1)
- ribbons (2)
- Robin (1)
- RSS feed (1)
- salivary (1)
- salivary cortisol (1)
- SEISMIC trial (1)
- Signifor (1)
- SILS (1)
- Sjogren's (1)
- sleep disturbance (1)
- Solu-Cortef (3)
- Spanish (1)
- Spironolactone (1)
- stereotactic (1)
- steroid (2)
- steroids (2)
- straie (4)
- stress (4)
- stretch marks (4)
- stroke (1)
- support (4)
- surgery (25)
- survey (1)
- symptoms (6)
- television (1)
- teriparatide (1)
- testing (4)
- Thankfulness (1)
- Thanksgiving (2)
- The Balancing Act (1)
- The Coffee Klatch (1)
- Thomas Addison (6)
- thyroid (12)
- topical steroid use (1)
- transsphenoidal (8)
- treatment (3)
- TSH (1)
- tumor (13)
- TV (1)
- Twitter (1)
- UFC (2)
- unilateral adrenalectomy (2)
- upgrade (1)
- video (10)
- Vitamin B12 deficiency (1)
- WEGO (1)
- weight (10)
- Wiki (1)
- X-ray (1)
- zebra (1)
- zebrafish (1)
- venous thromboembolism (HR 2.6, 95% CI 1.5-4.7)
- myocardial infarction (HR 3.7, 95% CI 2.4-5.5)
- stroke (HR 2.0, 95% CI 1.3-3.2)
- peptic ulcers (HR 2.0, 95% CI 1.1-3.6)
- fractures (HR 1.4, 95% CI 1.0-1.9)
- infections (HR 4.9, 95% CI 3.7-6.4).
Labels: adrenal, cortisol, Cushing's Syndrome, Pasireotide, pituitary, Signifor, surgery
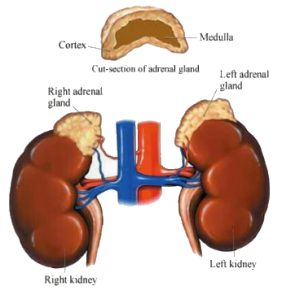
- the hypothalamus produces corticotropin-releasing hormones, which stimulate the pituitary gland.
- the pituitary gland, in turn, produces corticotropin hormones, which stimulate the adrenal glands to produce corticosteroid hormones.
- corticosteroid hormones
- hydrocortisone hormone - this hormone, also known as cortisol, controls the body's use of fats, proteins, and carbohydrates.
- corticosterone - this hormone, together with hydrocortisone hormones, suppresses inflammatory reactions in the body and also affects the immune system.
- aldosterone hormone - this hormone inhibits the level of sodium excreted into the urine, maintaining blood volume and blood pressure.
- androgenic steroids (androgen hormones) - these hormones have minimal effect on the development of male characteristics.
- epinephrine (also called adrenaline) - this hormone increases the heart rate and force of heart contractions, facilitates blood flow to the muscles and brain, causes relaxation of smooth muscles, helps with conversion of glycogen to glucose in the liver, and other activities.
- norepinephrine (also called noradrenaline) - this hormone has little effect on smooth muscle, metabolic processes, and cardiac output, but has strong vasoconstrictive effects, thus increasing blood pressure.
Journal of Clinical Endocrinology and Metabolism, 03/28/2013 Clinical Article
- The study used Cox–regression, and computed hazard ratios (HR) with 95% confidence intervals (95% CI).
- Morbidity was investigated in the three years before diagnosis; morbidity and mortality was assessed during complete follow–up after diagnosis and treatment.
- 343 CS patients and 34,300 controls were included. Mortality was twice as high in CS patients (HR 2.3, 95%CI 1.8–2.9) compared with controls.
- Patients with CS were at increased risk for venous thromboembolism (HR 2.6, 95%CI 1.5–4.7), myocardial infarction (HR 3.7, 95%CI 2.4–5.5), stroke (HR 2.0, 95%CI 1.3–3.2), peptic ulcers (HR 2.0, 95%CI 1.1–3.6), fractures (HR 1.4, 95%CI 1.0–1.9), and infections (HR 4.9, 95%CI 3.7–6.4).
- This increased multi–morbidity risk was present before diagnosis. Mortality and risk of myocardial infarction remained elevated during long–term follow–up.
- Mortality and risks for AMI, VTE, stroke and infections were similarly increased in adrenal and pituitary CS.
Do you blog? Want to get started?
Since April 8 is Cushing's Awareness Day, several people got their heads together to create the Second Annual Cushing's Awareness Blogging Challenge.
All you have to do is blog about something Cushing's related for the 30 days of April.
Robin designed this year's version of our "official logo" to put on your blogs.
In what ways have Cushing's made you a better person?
What have you learned about the medical community since you have become sick?
If you had one chance to speak to an endocrinologist association meeting, what would you tell them about Cushing's patients?
What would you tell the friends and family of another Cushing's patient in order to garner more emotional support for your friend?
Challenges with Cushing's? How have you overcome challenges? Stuff like that.
I have Cushing's Disease....(personal synopsis)
How I found out I have Cushing's
What is Cushing's Disease/Syndrome? (Personal variation, i.e. adrenal or pituitary or ectopic, etc.)
My challenges with Cushing's
Overcoming challenges with Cushing's (could include any challenges)
If I could speak to an endocrinologist organization, I would tell them...
. What would I tell others trying to be diagnosed? What would I tell families of those who are sick with Cushing's?
Treatments I've gone through to try to be cured/treatments I may have to go through to be cured.
What will happen if I'm not cured?
I write about my health because...
10 Things I Couldn’t Live Without.
My Dream Day.
What I learned the hard way
Miracle Cure. (Write a news-style article on a miracle cure. What’s the cure? How do you get the cure? Be sure to include a disclaimer)
Health Madlib Poem. Go to http://languageisavirus.com/cgi-bin/madlibs.pl and fill in the parts of speech and the site will generate a poem for you.
The Things We Forget. Visit http://thingsweforget.blogspot.com/ and make your own version of a short memo reminder. Where would you post it?
Give yourself, your condition, or your health focus a mascot. Is it a real person? Fictional? Mythical being? Describe them. Bonus points if you provide a visual!
5 Challenges and 5 Small Victories.
The First Time I...
Make a word cloud or tree with a list of words that come to mind when you think about your blog, health, or interests. Use a thesaurus to make it branch more.
How much money have you spent on Cushing's, or, How did Cushing's impact your life financially?
Why do you think Cushing's may not be as rare as doctors believe?
What is your theory about what causes Cushing's?
How has Cushing's altered the trajectory of your life? What would you have done? Who would you have been?
What three things has Cushing's stolen from you? What do you miss the most? What can you do in your Cushing's life to still achieve any of those goals? What new goals did Cushing's bring to you?
How do you cope?
What do you do to improve your quality of life as you fight Cushing's?
Your thoughts...?
Evaluation of depression, quality of life and body image in patients with Cushing’s disease
Posted by cushieNilufer Alcalar, Sedat Ozkan, Pinar Kadioglu, Ozlem Celik, Penbe Cagatay, Baris Kucukyuruk and Nurperi Gazioglu
Abstract
The aim of this study was to evaluate patients with Cushing’s disease (CD) who had undergone transsphenoidal surgery in terms of depression, quality of life (QoL), and perception of body image in comparison to healthy controls.
Forty patients with CD and 40 healthy controls matched for demographic characteristics were included in the study. The subjects were evaluated with the Beck depression inventory (BDI), the health survey-short form (SF-36) and the multidimensional body-self relations questionnaire (MBSRQ). Subgroups of the patients with CD were formed on the basis of remission status and BDI scores. In this study, QoL in the general health category and body image were lower in the patients with CD than in the healthy subjects. However, no differences in depression scores were found between the two groups.
When the CD group was evaluated according to remission rate, the mean BDI score was significantly higher in the CD patients without remission than in both the CD patients with remission and the healthy subjects (p = 0.04). However, the physical functioning, bodily pain and general health scores of the CD patients without remission on the SF-36 questionnaire were lower than in the CD patients in remission and the healthy subjects (p = 0.002, p = 0.04, p = 0.002, respectively). Fitness evaluation, health evaluation and body areas satisfaction scores of the MBSRQ were significantly different in the three groups (p = 0.003, p = 0.009 and p = 0.001, respectively). In this study, patients with CD were found to have lower QoL, lower body image perception and higher levels of depression compared to healthy controls, particularly if the disease is persistant despite surgery.
Keywords Cushing’s disease – Pituitary surgery – Depression – Quality of life – Body image
Labels: body image, Cushing's, depression, pituitary, quality of life, surgery
An older, but still useful, abstract:
J Clin Endocrinol Metab. 1986 Dec;63(6):1365-71.
Abstract
The therapeutic value of ketoconazole for long term treatment of patients with Cushing's syndrome was studied. Seven patients with Cushing's disease and one with an adrenal adenoma received 600-800 mg/day ketoconazole for 3-13 months. Plasma ACTH, cortisol, and dehydroepiandrosterone sulfate levels and urinary cortisol, 17-ketosteroid, and tetrahydro-11-deoxycortisol excretion were determined periodically during the treatment period.
Plasma ACTH and cortisol responses to CRH stimulation were determined before and during treatment. Rapid and subsequently persistent clinical improvement occurred in each patient; plasma dehydroepiandrosterone sulfate and urinary 17-ketosteroid and cortisol excretion decreased soon after the initiation of treatment, subsequently remaining normal or nearly so throughout the treatment period. Urinary tetrahydro-11-deoxycortisol excretion increased significantly. Plasma cortisol levels decreased. Plasma ACTH levels did not change, and individual plasma ACTH and cortisol increments in response to CRH were comparable before and during treatment. The cortisol response to insulin-induced hypoglycemia improved in one patient and was restored to normal in another.
The seven patients tested recovered normal adrenal suppressibility in response to a low dose of dexamethasone during ketoconazole treatment. Ketoconazole is effective for long term control of hypercortisolism of either pituitary or adrenal origin. Its effect appears to be mediated by inhibition of adrenal 11 beta-hydroxylase and 17,20-lyase, and it, in some unknown way, prevents the expected rise in ACTH secretion in patients with Cushing's disease.
Amir H. Hamrahian, MD, is a Staff member in the Department of Endocrinology, Diabetes and Metabolism at Cleveland Clinic's main campus, having accepted that appointment in 2005. Prior to that appointment, he was also a clinical associate there for nearly five years.
His clinical interests include pituitary and adrenal disorders.
Dr. Hamrahian received his medical degree from Hacettepe University in Ankara, Turkey, and upon graduation was a general practitioner in the provinces of Hamadan and Tehran, Iran. He completed an internal medicine residency at the University of North Dakota, Fargo, and an endocrinology fellowship at Case Western Reserve University and University Hospitals, Cleveland.
In 2003, he received the Teacher of the Year award from Cleveland Clinic's Department of Endocrinology, Diabetes and Metabolism. Dr. Hamrahian speaks three languages -- English, Turkish and Farsi -- and is board-certified in internal medicine as well as endocrinology, diabetes and metabolism. He is a member of the Endocrine Society, Pituitary Society and the American Association of Clinical Endocrinologists.
Some of the questions answered in this interview October 1, 2012 include (not in this order):
- Can you tell me a little about you endocrine practice and your experience with Cushing’s as part of your practice?
- What are some of biggest challenges you have in treating Cushing’s?
- How do you test cyclical/episodic Cushing's?
- Can someone with cyclical/episodic Cushing's take Korlym?
- I know that Cushing's patients (those that currently have it and/or are cured/in remission can have healthy pregnancies with the right care. How do doctors support this process? Through an endocrinologist and a high-risk ob/gyn? And what sort of treatment is given throughout the pregnancy to prevent hypercortisolism.
- While many patients have a successful long term result from surgery, there are just as many that don’t. Do you find that there are any particular challenges treating patients with Cushing’s disease when pituitary surgery has already failed?
- As I understand, you were an investigator in the clinical trial for Korlym, and I think you treated 4 patients. Did these patients all have a previous surgery that had failed?
- Many Cushing’s patients are trying to understand if they might be candidates for Korlym treatment, can you tell me a little history about the types of patients you treated with Korlym? I hear that not all patients can take Korlym. Which type of patient should not take it?
- Every past treatment for Cushing’s has always had the goal of lowering cortisol levels, but Korlym doesn’t lower cortisol levels, can you explain how it works?
- So, how do you judge success for a Cushing’s patient on Korlym?
- I lost copious amounts of hair while on Korlym, is this a known side effect?
- Are there any long term reproductive implications due to use of Korlym?
Listen to this interview at http://www.blogtalkradio.com/cushingshelp/2012/10/01/dr-amir-hamrahian-answers-our-questions or to the podcast by searching for Cushings in the iTunes podcast area or click here: http://itunes.apple.com/podcast/cushingshelp-cushie-chats/id350591438
Labels: adrenal, BLA, Cleveland Clinic, Cushing's, cyclic, Dr. Amir Hamrahian, Interviews, Korlym, pituitary, pregnancy
October 1, 2012 at 6:30 PM eastern, Dr. Amir Hamrahian will answer our questions about Cushing's, pituitary or adrenal issues and Korlym (mifepristone) in BlogTalkRadio at http://www.blogtalkradio.com/cushingshelp/2012/10/01/dr-amir-hamrahian-answers-our-questions
You may listen live at the link above. The episode will be added to the Cushing's Help podcast after the show is over. Listen to the podcasts by searching for Cushings in the iTunes podcast area or click here: http://itunes.apple.com/podcast/cushingshelp-cushie-chats/id350591438
Dr. Hamrahian has had patients on Korlym for about 4 years.
Please submit your questions below or email them to CushingsHelp@gmail.com before Sunday, September 30.
From Dr. Hamrahian's bio at http://my.clevelandclinic.org/staff_directory/staff_display.aspx?doctorid=3676
Amir Hamrahian, M.D.
(216) 444-6568
- Department:Endocrinology, Diabetes and Metabolism
- Location:Cleveland Clinic Main Campus
Mail Code F20
9500 Euclid Avenue
Cleveland, OH 44195 - Appointment:(216) 444-6568
- Desk:(216) 445-8538
- Fax:(216) 445-1656
- Department:Brain Tumor and Neuro-Oncology Center
- Location:Cleveland Clinic Main Campus
Mail Code R20
9500 Euclid Avenue
Cleveland, OH 44195 - Appointment:(216) 444-6568
- Desk:(216) 445-8538
- Fax:(216) 445-1656
- Surgeon:
- No
- Treats:
- Adults Only
( † Disclaimer: This search is powered by PubMed, a service of the U.S. National Library of Medicine. PubMed is a third-party website with no affiliation with Cleveland Clinic.)
Biographical Sketch
Amir H. Hamrahian, MD, is a Staff member in the Department of Endocrinology, Diabetes and Metabolism at Cleveland Clinic's main campus, having accepted that appointment in 2005. Prior to that appointment, he was also a clinical associate there for nearly five years.
His clinical interests include pituitary and adrenal disorders.
Dr. Hamrahian received his medical degree from Hacettepe University in Ankara, Turkey, and upon graduation was a general practitioner in the provinces of Hamadan and Tehran, Iran. He completed an internal medicine residency at the University of North Dakota, Fargo, and an endocrinology fellowship at Case Western Reserve University and University Hospitals, Cleveland.
In 2003, he received the Teacher of the Year award from Cleveland Clinic's Department of Endocrinology, Diabetes and Metabolism. Dr. Hamrahian speaks three languages -- English, Turkish and Farsi -- and is board-certified in internal medicine as well as endocrinology, diabetes and metabolism. He is a member of the Endocrine Society, Pituitary Society and the American Association of Clinical Endocrinologists.
Education & Fellowships
- Fellowship - University Hospitals of Cleveland
- Endocrinology
Cleveland, OH USA
2000 - Residency - University of North Dakota Hospital
- Internal Medicine
Fargo, ND USA
1997 - Medical School - Hacettepe University School of Medicine
- Ankara Turkey
1991
Certifications
- Internal Medicine
- Internal Medicine- Endocrinology, Diabetes & Metabolism
Specialty Interests
Awards & Honors
- Best Doctors in America, 2007-2008
Memberships
- Pituitary Society
- Endocrine Society
- American Association of Clinical Endocrinologists
- American Medical Association
Treatment & Services
- Radioactive Iodine Treatment
- Thyroid Aspiration
- Thyroid Ultrasound
Specialty in Diseases and Conditions
- Acromegaly
- Addison’s Disease
- Adrenal disorders
- Adrenal insufficiency
- Adrenal Insufficiency and Addison’s Disease
- Adrenal Tumors
- Adrenocortical Carcinoma
- Adrenoleukodystrophy (ALD)
- Amenorrhea
- Androgen Deficiency (Low Testosterone)
- Androgen Excess
- Calcium Disorders
- Carcinoid Syndrome
- Conn's Syndrome
- Cushing's Syndrome
- Empty sella
- Erectile Dysfunction
- Familial Multiple Endocrine Neoplasia
- Fasting hypoglycemia
- Flushing Syndromes
- Galactorrhea
- Goiter
- Growth hormone deficiency
- Growth hormone excess
- Gynecomastia
- Hirsutism
- Hyperaldosteronism
- Hyperandrogenism
- Hyperprolactinemia
- Hypertension - High Blood Pressure
- Hyperthyroidism
- Hypocalcemia
- Hypoglycemia
- Hypogonadism
- Hypoparathyroidism
- Hypophysitis
- Hypopituitarism
- Hypothyroidism
- Mastocytosis
- Menopause, Male
- Menstrual Disorders
- Paget's Disease
- Panhypopituitarism
- Parathyroid Cancer
- Parathyroid Disease and Calcium Disorders
- Pheochromocytoma
- Pituitary Cysts
- Pituitary Disorders
- Pituitary stalk lesions
- Pituitary Tumors
- Premenstrual Syndrome (PMS)
- Primary Hyperaldosteronism
- Primary Hyperparathyroidism
- Prolactin Excess States
- Prolactinoma
- Thyroid and pregnancy
- Thyroid Cancer
- Thyroid Disease
- Thyroid Nodule
Betul A. Hatipoglu MD*
Article first published online: 27 JUN 2012
DOI: 10.1002/jso.23197
Keywords:
Cushing's syndrome; adrenal carcinoma; virilization; hypercortisolism
Abstract
Cushing's syndrome (CS) results from prolonged exposure to elevated endogenous cortisol. Majority of cases are caused by ACTH, pituitary, or ectopic origin. Primary adrenal hypersecretion is 15–20% caused by adenomas, carcinomas (ACC), and rarely by nodular adrenocortical disease. CS presents with all typical features.
Commonly recommended initial testing are urinary free cortisol, late-night salivary cortisol, and 1-mg overnight dexamethasone suppression test (DST). Imaging is the key to diagnosis. CS continues to pose diagnostic and therapeutic challenges; life-long follow-up is mandatory.
J. Surg. Oncol © 2012 Wiley Periodicals, Inc.
Read this article at Wiley Online Publications
Gabriel Zada, Amir Tirosh, Ursula B. Kaiser, Edward R. Laws and Whitney W. Woodmansee
Department of Neurosurgery (G.Z., E.R.L.) and Division of Endocrinology, Diabetes, and Hypertension (A.T., U.B.K., W.W.W.), Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts 02115
Address all correspondence and requests for reprints to: Gabriel Zada, M.D., 15 Francis Street, PBB3, Boston, Massachusetts 02115. E-mail: gzada@usc.edu.
Abstract
Case Illustration: A 33-yr-old woman with Cushing’s disease underwent successful surgical resection of a pituitary adenoma and developed IIH 11 months later after inadvertent withdrawal of oral glucocorticoids.
Methods: A review of the literature was conducted to identify previous studies pertaining to IIH in association with neuroendocrine disease, focusing on reports related to HPA axis dysfunction.
Results: A number of patients developing IIH due to a relative deficiency in glucocorticoids, after surgical or medical management for Cushing’s disease, withdrawal from glucocorticoid replacement, or as an initial presentation of Addison’s disease, have been reported. Hypotheses regarding the underlying pathophysiology of IIH in this context and, in particular, the role of cortisol and its relationship to other neuroendocrine and inflammatory mediators that may regulate the homeostasis of cerebrospinal fluid production and absorption are reviewed.
Conclusion: In a subset of patients, dysfunction of the HPA axis appears to play a role in the development of IIH. Hormonal control of cerebrospinal fluid production and absorption may be regulated by inflammatory mediators and the enzyme 11ß-hydroxysteroid dehydrogenase type 1. Further study of neuroendocrine markers in the serum and cerebrospinal fluid may be an avenue for further research in IIH.
Read the entire article at http://jcem.endojournals.org/content/95/11/4850.full